Annovis Bio Inc. (NYSE: ANVS)
Tip on: June 27, 2024

Recent Achievements
Annovis Bio continues to advance its mission of developing transformative therapies for neurodegenerative disorders. The company recently announced statistically significant data from its Phase II/III Alzheimer’s study, demonstrating the potential of its lead drug candidate, buntanetap, to improve cognition in patients with early Alzheimer’s Disease (AD). Additionally, the company has released promising data from its Phase III study of buntanetap in patients with early Parkinson’s Disease (PD), which highlights significant advancements in both cognitive and motor functions. These milestones underscore the expanding therapeutic reach of buntanetap.
Investment Considerations
- Unique Market Position: Annovis Bio is uniquely positioned as the only company developing a drug for both AD and PD that inhibits multiple neurotoxic proteins simultaneously.
- Strong Clinical Results: Buntanetap’s Phase II/III data shows significant cognitive improvement in early AD patients, and the recent Phase III data in PD patients further validates its broad therapeutic potential.
- Strategic Growth Plans: With recent successful trial results, Annovis Bio is poised for future growth, supported by strong patent protections and upcoming clinical trials.
- Significant Market Need: As the prevalence of neurodegenerative diseases continues to rise, the demand for effective treatments like buntanetap remains critical.
Annovis Bio Inc. Overview
Annovis Bio Inc. (NYSE: ANVS) is a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative disorders such as AD and PD. Annovis Bio stands out by developing a drug that targets multiple neurotoxic proteins simultaneously, aiming to restore axonal and synaptic activity. This innovative approach addresses both the cognitive decline in AD and the motor dysfunction in PD, making Annovis a unique player in the neurodegeneration space.
Lead Drug Candidate: Buntanetap
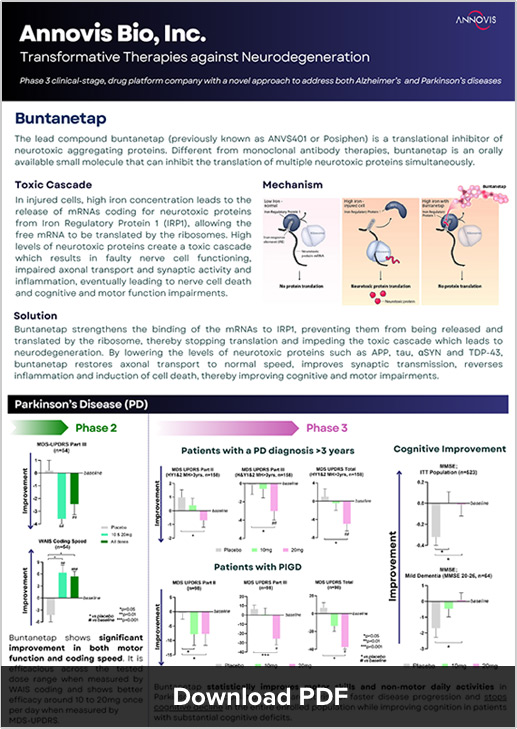
Buntanetap (formerly known as Posiphen) targets neurodegeneration by inhibiting the formation of multiple neurotoxic proteins, including amyloid beta, tau, alpha-synuclein, and TDP43. This multifaceted inhibition improves synaptic transmission and axonal transport, reduces neuroinflammation, and protects nerve cells from dying. Unlike monoclonal antibody therapies, buntanetap is an orally available small molecule capable of inhibiting multiple neurotoxic proteins at once, positioning it as a comprehensive solution for neurodegenerative diseases.
In a recent Phase II/III Alzheimer’s study, buntanetap demonstrated statistically significant efficacy. Patients with early AD showed a significantly higher rate of improvement in ADAS-Cog 11 scores across all treatment doses compared to placebo, with a 3.3 point improvement compared to 0.3 for placebo (p < 0.01). Plasma Tau protein levels also reduced, consistent with previous Phase II biomarker data, further validating buntanetap’s mechanism of action.
Similarly, in the recently completed Phase III study of buntanetap in patients with early PD, buntanetap significantly improved disease-related daily non-motor and motor functions in Parkinson’s patients who had a diagnosis over 3 years as well as improved cognition in all PD patients. It further underscores buntanetap’s potential as a transformative therapy.
Market Opportunity
The aging population presents a significant market opportunity, with nearly 7 million Americans currently suffering from Alzheimer’s Disease (AD), a figure projected to rise to almost 13 million by 2050 (Alzheimer’s Association) (Republican Policy Committee). Additionally, approximately 1.2 million people in the U.S. have Parkinson’s Disease (SingleCare).
The economic burden of Alzheimer’s is immense, with care costs expected to reach $360 billion in 2024 and escalate to nearly $1 trillion annually by 2050. The need for effective, comprehensive treatments like Buntanetap is more critical than ever.
Company Highlights
- Innovative Therapeutic Approach: Annovis Bio uniquely targets multiple neurotoxic proteins, aiming to restore nerve cell health and improve cognitive and motor function in AD and PD patients.
- Robust Clinical Data: Phase II/III studies show significant improvements in cognitive function and biomarker levels in early AD patients.
- Groundbreaking Clinical Insights: Recent Phase III data in Parkinson’s Disease patients demonstrates significant improvements in motor and cognitive functions.
- Upcoming Phase III Trials: Plans are underway for an 18-month Phase III trial focusing on biomarker-positive early AD patients, designed to further validate buntanetap’s disease-modifying potential.
- Capital Efficiency: Annovis Bio is capital-efficient, with zero debt and multiple global patents extending into the 2040s.
Management Team
- Maria L. Maccecchini, Ph.D. – Founder, President, CEO, and Executive Board Member, founded Annovis Bio in May 2008 with the mission to develop better therapeutics for Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. She has previously been a partner and director at two angel groups, Robin Hood Ventures and MidAtlantic Angel Group, and founded Symphony Pharmaceuticals/Annovis, which was sold to Transgenomic in 2001. Her extensive experience includes roles such as General Manager at Bachem Bioscience and Head of Molecular Biology at Mallinckrodt. Dr. Maccecchini holds a Ph.D. in biochemistry from the Biocenter of Basel, with postdoctoral work at Caltech and the Roche Institute of Immunology.
- Cheng Fang, Ph.D. – Senior VP of Research and Development, is an accomplished neuroscientist with two decades of experience in neurodegenerative diseases. She has a successful track record of scientific publications and contributions, coupled with extensive pre-clinical and clinical development experience. Dr. Fang has been instrumental in advancing the understanding of neurodegenerative disease mechanisms and developing therapeutic strategies.
- Michael Christie, Ph.D. – VP of Process Chemistry, has over 40 years of experience in the pharmaceutical industry, focusing on process chemistry R&D, pilot plant production, and GMP operations. He has held senior management positions at companies such as SmithKline, Rhodia, Teva, and Cephalon, and founded a contract process R&D service company, which was later acquired by ChiRex. Dr. Christie is co-author or co-inventor on several publications and patents. He earned his BS in chemistry from the University of Michigan and his doctorate from MIT.
- Melissa Gaines – Senior VP of Clinical Operations, is an accomplished clinical research professional with over 20 years of experience across academia, contract research organizations, and pharmaceutical companies. She has proven abilities in monitoring and managing Phase I to IV clinical trials, specializing in CNS disorders and extending to a broad range of therapeutic indications. Her CNS experience spans from small Phase I and II studies to large global Phase III trials in Alzheimer’s disease, Parkinson’s disease, sleep disorders, and various psychiatric diseases in both adult and pediatric populations. In her current role, she oversees and supports all clinical project activities, driving operational success and ensuring high-quality clinical outcomes.
Additional Resources